Radical-Free Digital Light Processing 3D Printing of Hydrogels Using a Photo-Caged Cyclopentadiene Diels–Alder Strategy
Takashi Kaneko, Ronnie V. Garcia, Allison L. Chau, Angela A. Pitenis, Sijia Huang, Bryan D. Moran, Sophia J. Bailey, Craig J. Hawker, Javier Read de Alaniz
Adv. Funct. Mat. August 2025
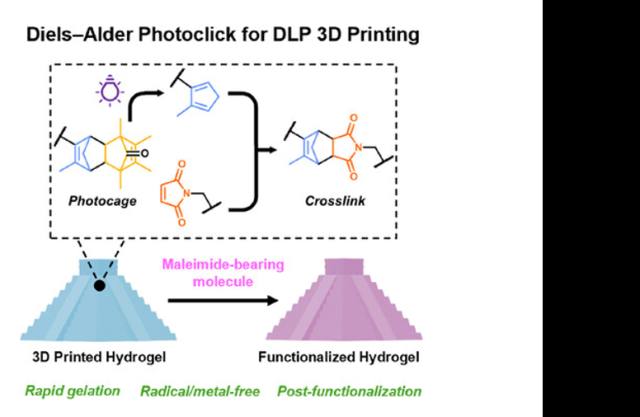
Abstract: Light-controlled chemical reactions have driven major advances in photo-patterning and 3D printing, particularly for applications requiring spatially and temporally resolved control over soft material assembly. Synthetic hydrogels, which mimic the extracellular matrix, are widely used in 3D cell culture, drug delivery, and soft device platforms. While radical-mediated photo-crosslinking dominates digital light processing (DLP) bioprinting, the high reactivity of free radicals can compromise cell/biomolecule stability and functional group integrity. This issue has led to a shift toward radical-free, light-controlled crosslinking. In this study, a novel approach is presented for the first demonstration of radical-free aqueous photo-resins for DLP printing, utilizing photo-caged cyclopentadiene (Cp) moieties and maleimide click partners. Upon 365 nm light exposure, uncaged Cp reacts rapidly with maleimide groups via a Diels–Alder cycloaddition, enabling fast gelation and high-fidelity DLP printing. The two-component resin system offers tunable mechanical properties and yields printed features with submillimeter fidelity. Critically, the resulting materials retain unreacted functional handles, enabling spatially resolved post-functionalization with small molecules in the complete absence of radicals. This platform not only provides a robust and orthogonal alternative to traditional photo-resins, but also opens new avenues for biofabrication, adaptive soft materials, and 4D-printed systems where chemical precision and compatibility are paramount.