Selective electrochemical degradation of bottlebrush elastomers
Kaitlin R. Albanese, Parker T. Morris, Brian Roehrich, Javier Read de Alaniz, Craig J. Hawker, Christopher M. Bates
J. Polym. Sci. July 2024
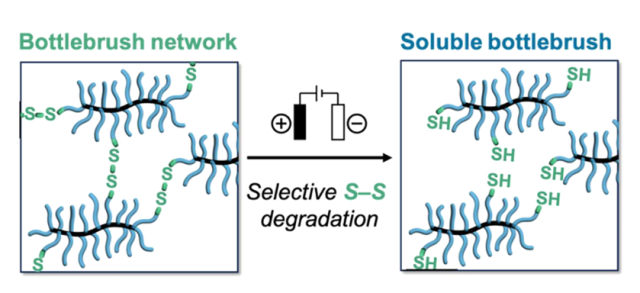
Abstract: We introduce a simple synthetic strategy to selectively degrade bottlebrush networks derived from well-defined poly(4-methylcaprolactone) (P4MCL) bottlebrush polymers. Functionalization of the hydroxyl groups present at the terminal ends of P4MCL side chains with α-lipoic acid resulted in bottlebrush polymers having a range of molecular weights (Mn = 45–2200 kg mol−1) and a tunable number of reactive dithiolane chain ends. These functionalized chain ends act as efficient crosslinkers due to radical ring-opening of the dithiolane rings under UV light. The resulting redox-active disulfide crosslinks enable mild electrochemical or chemical degradation of the SS crosslinks to regenerate the starting bottlebrush polymer. P4MCL side chains and the disulfides can be degraded simultaneously using harsher reducing conditions. This combination of bottlebrush architecture with facile disulfide crosslinking presents a versatile platform for preparing highly tunable elastomers that undergo controlled degradation under mild conditions.