Neighboring Group Participation in Ionic Covalent Adaptable Networks
Lindsay L. Robinson, Eden S. Taddese, Jeffrey L. Self, Christopher M. Bates, Javier Read de Alaniz, Zhishuai Geng, and Craig J. Hawker
Macromolecules 2022, 55, 21, 9780–9789
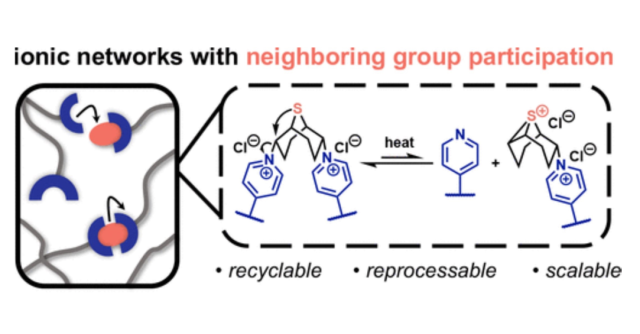
Abstract: Covalent adaptable networks (CANs) typically require external catalysts for efficient cross-linker exchange, which can limit network reprocessability due to catalyst leaching and degradation. In this study, catalysts were avoided by using a bicyclo[3.3.1]nonane bis-alkyl halide cross-linker with sulfur-atom neighboring group participation (NGP) to increase the rate of bond exchange. Stress relaxation analyses demonstrate that the resultant pyridine-based network has an Arrhenius dependence on viscous flow at elevated temperatures (130–170 °C), which arises from SN1 transalkylation exchange. This thermally mediated cross-link interchange and associated flow behavior enabled reprocessing of the ionic networks over multiple damage and repair cycles. Additionally, these NGP-based CANs are chemically recyclable, allowing for recovery of the pyridyl-based polymer starting material, which comprises >90 wt % of the parent network. The dual thermal and chemical recycling potential of this catalyst-free CAN platform addresses key criteria for designing thermosets with extended lifecycles.